Jordan Lake Bird Counts—Part 4, Species That Are Increasing
Author: Norm Budnitz
[Part 1 in this series of articles covered background information about the Jordan Lake bird counts and discussed the long-term data for several species of birds that are closely tied to the lake itself. Part 2 looked at the long-term data for one family of birds, the woodpeckers. Part 3 discussed issues concerning species in decline.]
The populations of several species we have been monitoring for 40 years in the Jordan Lake count circle have been increasing. Is this good news? Are more birds always better? This article examines the data for some of these species and tries to identify patterns that may help us understand what has been happening.
Introductions and Reintroductions
We humans tend to ‘mess with Mother Nature,’ as the saying goes. We introduce species to areas where they have never been before, and we re-introduce species to areas where they have been extirpated. We even introduce species for our own uses.

Figure 1. House Finch (photograph, Norm Budnitz)
House Finch (Haemorhous mexicanus)
House Finches were originally birds of dry, open habitats in the southwestern part of the United States and Mexico. In 1939, a few individuals were released from a pet store in New York City (Badyaev, et al., 2020). These birds bred, and by the mid-1940s, a small population became established on Long Island and surrounding areas. In the ensuing decades, House Finch populations expanded rapidly throughout the eastern half of the United States.
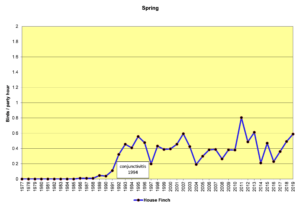
Figure 2. SBC, House Finch
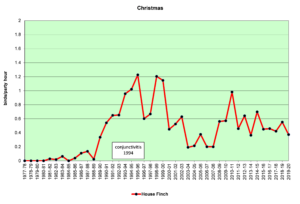
Figure 3. CBC, House Finch
Figures 2 and 3 show that House Finches were first recorded on our spring (SBC) and Christmas (CBC) bird counts in the mid-1980s. In 1994, an eye infection (conjunctivitis) appeared in House Finches in Washington, D.C. The disease may have spread from poultry, where it is common. This illness, often lethal in finches, soon spread throughout populations in the east, including North Carolina. As can be seen in the graphs above, our local finch population declined in the mid-1990s and has fluctuated a bit ever since. The disease seems to take its toll most heavily in the winter. Our CBCs often show increases in breeding birds because of the addition of young birds from the previous breeding season. By spring, in stable populations, the numbers return to their usual levels. That seems to have happened with House Finches, so perhaps, they have developed some immunity to the disease over time.

Figure 4. Wild Turkey (photograph, Norm Budnitz)
Wild Turkey (Meleagris gallopavo)
Wild Turkeys were found throughout North Carolina when Europeans first came to North America. However, in the 1800s, market hunting and deforestation decimated the turkey population; by the early 1900s their numbers dropped to near zero (McRoberts, et al., 2020; North Carolina Wildlife Resources Commission, 2020). Restoration of turkeys began in the mid-1900s, and since 1953 more than 6,000 birds have been released in North Carolina, mostly in the western part of the state and in counties along the Virginia border, but not in our immediate area. Statewide restoration efforts ended in 2005, about the time that we started seeing turkeys on our bird counts. The statewide population has increased from about 2,000 birds in 1970 to more than 265,000 birds in 2015.
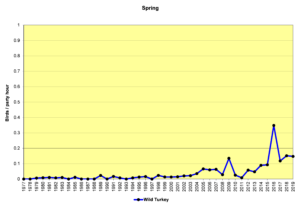
Figure 5. SBC, Wild Turkey
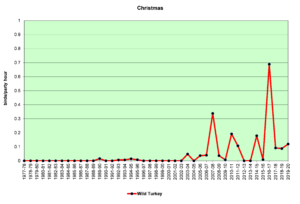
Figure 6. CBC, Wild Turkey
Figures 5 and 6 show that sightings of Wild Turkeys in the Jordan Lake Count Circle were rare until about 2005. Though they have not been seen in large numbers in any given year, they have been reported on almost every count in the past 10 years. These birds have probably spread from surrounding areas where they have been reintroduced. When turkeys forage in open habitats—grasslands or weedy fields—they can be relatively easy to see, because they are large. In forested habitats, they can be extremely difficult to see even at remarkably close range if they are quiet. If they do take flight to leave the scene, they do so with an extremely loud flapping of wings that will cause the human heart to beat dramatically and the accompanying adrenalin rush may take several minutes to subside.

Figure 7. Canada Goose (photograph, Vern Bothwell)
Canada Goose (Branta canadensis)
Canada Geese in piedmont North Carolina are more land birds than water birds. They are grazers, happily feeding on lawns and golf courses, and moving to water mostly for safety at night and during the breeding season. Such was not always the case. In the middle of the 1900s, Canada Geese were generally seen only in winter, when migratory birds returned south from their breeding grounds further north. But in the mid-1900s, geese were introduced (or reintroduced) in the midwestern and southern states. The genetics are murky, but it seems that many of the introduced birds came from sedentary, perhaps semi-domesticated populations. These birds, which do not migrate, found lots of amenable habitat with abundant resources. The result has been a population explosion. The sounds of a skein of geese flying overhead in October no longer indicate the return of wild birds from the far north. Those birds may just be your neighborhood geese, moving from one lawn to another.

Figure 8. SBC, Canada Goose
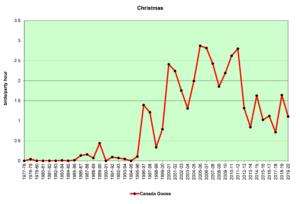
Figure 9. CBC, Canada Goose
Figures 8 and 9 show that Canada Geese began to appear in our count circle in significant numbers in the mid-1990s. A rapid increase in breeding birds (Fig. 8) may now be leveling off. The higher winter numbers (Fig. 9) probably reflect the young birds from the breeders of the previous spring. Adult geese are large, powerful birds with no real predators other than humans. They have thrived in our suburban areas where hunting is not allowed. If their numbers are leveling off, as our data indicate, perhaps they have reached the carrying capacity of our suburban resources. Bird counts over the next decade may help us to understand what is happening.
Range Expansions
Sometimes, species that were once uncommon in an area become more common as a result of range expansion from populations elsewhere. Following are some examples in our area. In each case, the species in question are compared to closely related species that were already present.

Figure 10. Turkey Vulture (photograph, Joe Donahue)

Figure 11. Black Vulture (photograph, Vern Bothwell)
Turkey Vulture (Cathartes aura)
Black Vulture (Coragyps atratus)
These two vulture species are closely related, but they have some interesting differences that are significant. Both species eat mostly carrion. Turkey Vultures find their food by soaring overhead and using visual and olfactory cues to detect carcasses. Unlike most bird species, Turkey Vultures have a very well-developed sense of smell (Buckley, 2020). They have been caught in concealed traps that were baited only with scent, and they often approached those traps from a downwind direction. Black Vultures, on the other hand, hunt by sight. They find food visually; however, they also watch Turkey Vultures and will move in aggressively and take over a carcass the Turkey Vultures have found.
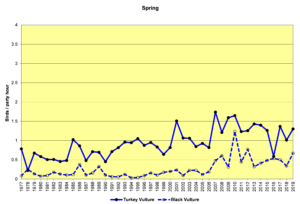
Figure 12. SBC, Turkey Vulture and Black Vulture
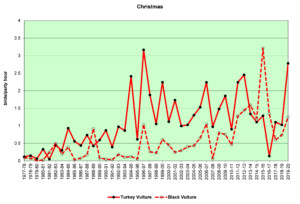
Figure 13. CBC, Turkey Vulture and Black Vulture
Finding vultures on our counts can be a hit or miss operation. They do not usually start flying until well after the sun has come up and begins heating the land surface, creating thermals for the vultures to use to get a free ride up to soaring/foraging altitude. In rainy weather they may not fly at all. On the other hand, they often roost communally, sometimes both species together. The result can be seen in the large fluctuations in the data from year to year, especially in winter (Figs. 12 and 13). The graphs show that both Turkey Vultures and Black Vultures have been on the increase over the decades, and that Turkeys have almost always outnumbered Blacks. However, it appears that Blacks may be increasing faster in recent years while the population growth of Turkeys may have leveled off.
Both vulture species are year-round residents throughout South America and southern North America, and both are somewhat migratory. Some individuals reside in our area year-round, while others, particularly Turkey Vultures, migrate north to breed, returning in the winter months (Buckley, 2020; Kirk, et al., 2020). Black Vultures do not move quite as much, and they have not extended their range as far north as Turkey Vultures. The observed range expansion and increase in numbers are probably influenced by human activities such as the creation of large landfill sites and increases in road kills resulting from increased development of roads and highways.

Figure 14. American Crow (photograph, Brendan Klick)

Figure 15. Fish Crow (photograph, Brendan Klick)
American Crow (Corvus brachyrhynchos)
Fish Crow (Corvus ossifragus)
These two species of crows are almost indistinguishable, except by voice. Fish Crows are a bit smaller, but this is not a reliable field mark. The relative lengths of two of their primaries are characteristic. The labels added to Brendan Klick’s excellent photographs above show that P9 is about the same length as P5 in American Crows, while P9 is longer than P5 in Fish Crows. Of course, observing these feathers on a flying bird in the field is all but impossible.
On the other hand, in spring, when both species are vocal, they can be relatively easy to identify, if they call. The drawn out “caw” of an American Crow is quite different from the shorter “uh uh” or “car car” of a Fish Crow. However, juvenile American Crows can sound just like Fish Crows, so summer identification can be trickier. And in winter, when both species tend to be much less vocal, it can be impossible to tell them apart in the field.
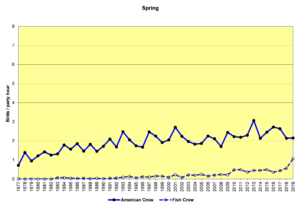
Figure 16. SBC, American Crow and Fish Crow
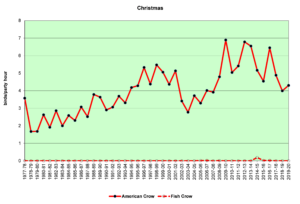
Figure 17. CBC, American Crow and Fish Crow
Figures 16 and 17 show that American Crows have been increasing slowly but steadily for the past four decades. Fish Crows were rare during this period until the early 2000s, when they became more regular in the spring and summer months, though they are far outnumbered by American Crows. Prior to the 2000s, Fish Crows in North Carolina were considered to be a coastal or tidewater species. As Figure 17 shows, they have been all but absent in the Piedmont when we do our CBCs, probably withdrawing east, back toward the coast in December and January. However, it is possible that they have simply not been counted if they have been silent. In general, when a crow is seen but not heard, it is counted as an American Crow—the default crow. This could bias our data to some degree.
It is difficult to speculate with any certainty why these species have been increasing. Both species tolerate and even take advantage of human population centers. Both species readily forage at landfills and parking lots of fast food restaurants. And Fish Crows may be attracted to the large reservoirs humans have created in the area (Jordan and Falls Lakes).
Human ‘Habitats’
Several species in our area seem to respond well to the alterations humans have made to the environment. Residential areas with trees and lawns, ornamental plantings, bird feeders, water features, and nest boxes are all attractive to some species at the expense of others that require larger forest tracts, shrubby second growth, or extensive grasslands. Red-bellied Woodpeckers, as discussed in Part 2 of these articles, is a typical example. The species discussed below also seem to fit this pattern.

Figure 18. Carolina Chickadee (photograph, Norm Budnitz)

Figure 19. Tufted Titmouse (photograph, Norm Budnitz)
Carolina Chickadee (Poecile carolinensis)
Tufted Titmouse (Baeolophus bicolor)
Chickadees and titmice are “leaders of the pack.” In the Piedmont region of North Carolina, they are non-migratory, year-round, permanent residents. In the spring, they pair up to lay eggs and raise young, but by mid-summer they congregate in small, multi-species flocks and move around their home range in search of resources. Other species join these roving bands, especially in winter—for example, White-breasted Nuthatches, Downy Woodpeckers, Brown Creepers, Ruby-crowned and Golden-crowned Kinglets. Chickadees and titmice seem to explore every nook and cranny in the natural environment—searching under leaves, probing into tree bark crevices, poking into dead flower stalks—but also in human constructions—searching under eaves, probing into siding, poking into forgotten laundry on a clothes line. And of course, when humans put up bird feeders, chickadees and titmice are usually the first species to find them.
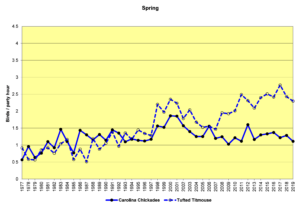
Figure 20. SBC, Carolina Chickadee and Tufted Titmouse
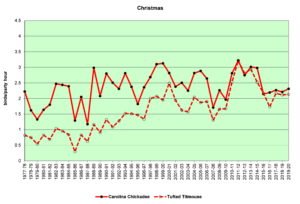
Figure 21. CBC, Carolina Chickadee and Tufted Titmouse
Figures 20 and 21 show that the populations of both species are increasing in the Jordan Lake bird count circle. This growth parallels the increase in numbers and the expansion in range of humans in this area. Parks and campgrounds, residential communities, and small commercial zones all create habitats that provide resources these birds need. These graphs indicate that while the populations of both species are increasing, titmice numbers seem to be growing faster than chickadee numbers. In winter (Fig. 21, CBC), chickadees almost always outnumber titmice in any given year, but titmice are clearly gaining. In spring (Fig. 20, SBC), chickadees were more common until the 1990s, when titmice took the lead and have outnumbered chickadees ever since.
The following speculations by the author are just that—speculations, not definitive explanations. The intent is to pique interest and perhaps experimentation and further analysis by others. Two questions to consider: 1) Overall, why are titmice increasing more rapidly than chickadees? 2) What happens over the course of a year such that the number advantage of chickadees in December becomes a number advantage for titmice by May?
- Regarding the first question, changes in land use patterns by humans could be affecting the two species differently. Chickadees and titmice may differ in certain subtle ways in terms of food preferences, nest site selection, interactions with other bird species, and so forth. Any or all of these differences could be influenced by changes wrought by humans. The number of people living in the Triangle area (Raleigh, Durham, and Chapel Hill) has increased dramatically during these four decades. Growing numbers of housing developments and shopping malls, development of industrial parks, and decrease of natural areas are all factors that might affect these two species differently.
- Regarding the second question, it could be that the relative numbers recorded is an artifact of the techniques used by the counters. Chickadees and titmice may breed at different times. For example, during the spring count period (the first week in May), if chickadees breed earlier, they may be incubating eggs and singing and moving about less conspicuously, while titmice may be singing vociferously, establishing territories and attracting mates.
- Climate change since the early 1990s may have reached a threshold that influenced the winter hardiness of one or the other or both of these species. For example, perhaps titmice are more subject to winter kill than chickadees at certain temperatures. If winters are now milder, this might allow for greater relative survival of titmice and therefore larger numbers reported in the spring.
- Or the opposite could be true. Milder winters might have a negative impact on chickadees, making them more susceptible to certain diseases that decrease their numbers.
- If there is an advance in breeding dates due to climate change, this might affect which species is singing when and therefore affect the ‘apparent’ numbers of individuals and subsequently their reported numbers.

Figure 22. Brown-headed Nuthatch (photograph, Norm Budnitz)

Figure 23. White-breasted Nuthatch (photograph, Norm Budnitz)
Brown-headed Nuthatch (Sitta carolinensis)
White-breasted Nuthatch (Sitta carolinensis)
Though closely related, these two species have strong preferences for quite different habitats. Brown-headed Nuthatches are birds of mature pine forests (Slater, et al., 2020). Throughout its range in southeastern North America , this nuthatch is always associated with pines, though the species of pine varies, depending on local conditions. In our area, the most common species is Loblolly Pine (Pinus taeda). Loblolly stands are regularly managed—clear cut, reseeded, allowed to mature, and then clear cut again. Loblollies can grow to 50 feet tall in just 20 years, so depending on how they will be used (wood pulp or lumber), the harvesting cycle may be just a few decades long.
White-breasted Nuthatches prefer mature deciduous forests or mixed deciduous/conifer forests. These forests take longer to develop and are typically less common in our area. Perhaps this is why White-breasted Nuthatches are less common in our area than Brown-headed Nuthatches.
About a decade ago, Audubon North Carolina began a program to install 10,000 nest boxes specifically for Brown-headed Nuthatches (Audubon North Carolina, 2020). These nuthatches typically excavate holes in dead snags or use holes made by woodpeckers. Audubon NC became concerned that climate change and human activities could potentially threaten these birds.
Figure 24 shows that the populations of both species are increasing. It is difficult to speculate about how the interplay among forest growth and harvesting, human incursions into their habitats, and the Audubon NC program might be affecting these species.
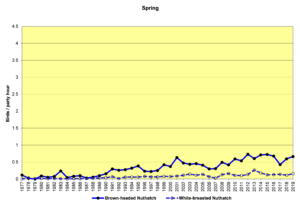
Figure 24. SBC, Brown-headed Nuthatch and White-breasted Nuthatch
Other Species
The alterations humans have made to the environment may be influencing population increases in other species as well. The following spring count (SBC) graphs show the data for several of these species. The Christmas count (CBC) graphs would be similar. I will let the graphs speak for themselves, rather than restate the factors listed above in the introduction to this section. All these graphs use the same vertical scale of 0 to 4.5 birds/party hour (the same as for the chickadee and titmouse and nuthatch graphs) to show their abundance relative to each other.

Figure 25. Carolina Wren (Thryothurus ludovicianus) (photograph, Norm Budnitz)
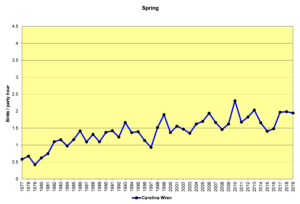
Figure 26. SBC, Carolina Wren

Figure 27. Eastern Bluebird (Sialia sialis) (photograph, Bill Majoros)
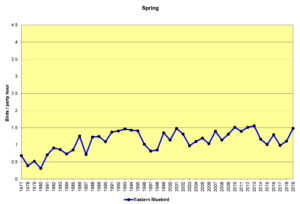
Figure 28. SBC, Eastern Bluebird

Figure 29. Northern Mockingbird (Mimus polyglottos) (photograph, Norm Budnitz)
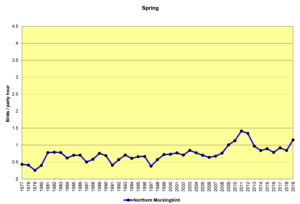
Figure 30. SBC, Northern Mockingbird

Figure 31. Chipping Sparrow (Spizella passerine) (photograph, Norm Budnitz)
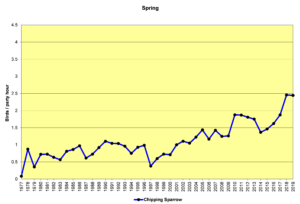
Figure 32. SBC, Chipping Sparrow

Figure 33. Northern Cardinal (Cardinalis cardinalis) (photograph, Tom Driscoll)
Figure 34. SBC, Northern Cardinal
Discussion
The species mentioned in this article all show signs of increasing population sizes in piedmont North Carolina. And these increases have all been mediated in one way or another by human activities—introduction or reintroduction by humans, global warming, and extensive increases in residential and commercial areas. In fact, this can be said for the birds mentioned in all four of these articles. While increases in some species are aided by these human activities, decreases in others appear to be the result of these same activities. The 40-plus years of data we have gathered, and will continue to gather, should help us understand these long-term changes.
Literature Cited
Audubon North Carolina (2020). https://nc.audubon.org/conservation/make-little-room-brown-headed-nuthatch
Badyaev, A. V., V. Belloni, and G. E. Hill (2020). House Finch (Haemorhous mexicanus), version 1.0. In Birds of the World (A. F. Poole, Editor). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.houfin.01
Buckley, N. J. (2020). Black Vulture (Coragyps atratus), version 1.0. In Birds of the World (A. F. Poole and F. B. Gill, Editors). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.blkvul.01
Grubb Jr., T. C. and V. V. Pravosudov (2020). White-breasted Nuthatch (Sitta carolinensis), version 1.0. In Birds of the World (A. F. Poole, Editor). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.whbnut.01
Kirk, D. A. and M. J. Mossman (2020). Turkey Vulture (Cathartes aura), version 1.0. In Birds of the World (A. F. Poole and F. B. Gill, Editors). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.turvul.01
McGowan, K. J. (2020). Fish Crow (Corvus ossifragus), version 1.0. In Birds of the World (A. F. Poole and F. B. Gill, Editors). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.fiscro.01
McRoberts, J. T., M. C. Wallace, and S. W. Eaton (2020). Wild Turkey (Meleagris gallopavo), version 1.0. In Birds of the World (A. F. Poole, Editor). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.wiltur.01
Mowbray, T. B., C. R. Ely, J. S. Sedinger, and R. E. Trost (2020). Canada Goose (Branta canadensis), version 1.0. In Birds of the World (P. G. Rodewald, Editor). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.cangoo.01
North Carolina Wildlife Resources Commission (2020). https://www.ncwildlife.org/Learning/Species/Birds/Wild-Turkey#2489428
Slater, G. L., J. D. Lloyd, J. H. Withgott, and K. G. Smith (2020). Brown-headed Nuthatch (Sitta pusilla), version 1.0. In Birds of the World (A. F. Poole, Editor). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.bnhnut.01
Verbeek, N. A. and C. Caffrey (2020). American Crow (Corvus brachyrhynchos), version 1.0. In Birds of the World (A. F. Poole and F. B. Gill, Editors). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.amecro.01
« Back to All Press